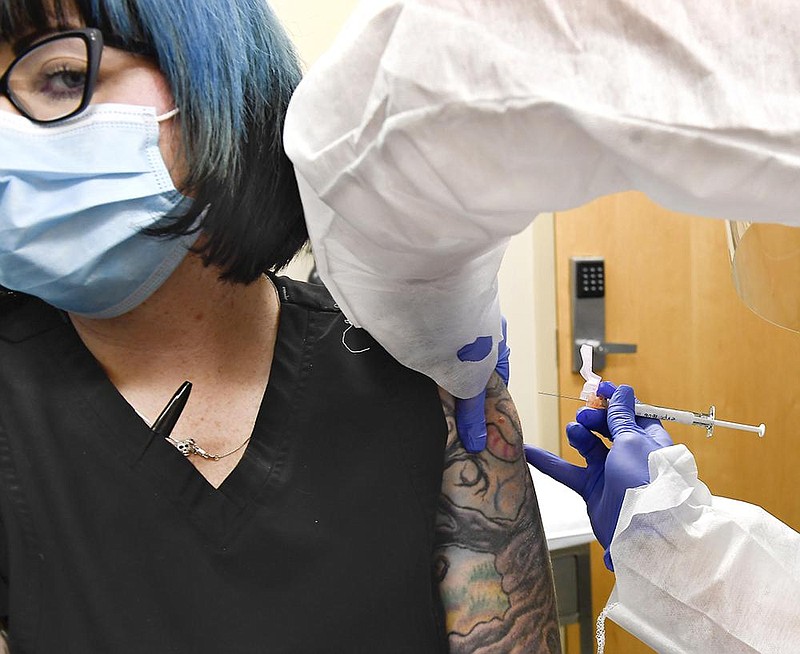
A volunteer Monday in Savannah, Ga., became the first participant in a vast human experiment to test the effectiveness of an experimental coronavirus vaccine.
The vaccine is being developed by the biotechnology company Moderna in collaboration with the National Institutes of Health.
It marks the official launch of the first in a series of U.S. clinical trials to test experimental vaccines in 30,000 participants, half receiving the medicine and half receiving a placebo.
Also, pharmaceutical giant Pfizer announced that it was initiating a 30,000-person vaccine trial at 120 sites globally.
[Video not showing up above? Click here to watch » https://www.youtube.com/watch?v=O_USQsc7KfE]
"We are participating today in the launching of a truly historic event in the history of vaccinology," Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, said at a news conference.
He noted that the United States has never moved faster to develop a vaccine, from basic science to a large Phase 3 trial designed to test safety and effectiveness.
Fauci predicted that researchers likely will be able to tell whether the Moderna vaccine is effective by November or December, though he said it is a "distinct possibility" that an answer could come sooner.
Pfizer officials have said the company expects to be able to seek regulatory authorization or approval by October.
The glimmer of hope came even as Google, in one of a major employer's gloomiest assessments of the coronavirus' staying power, decreed that most of its 200,000 employees and contractors should work from home through June of next year -- a decision that could influence other big companies.
Meanwhile, in Washington, the Trump administration disclosed that national security adviser Robert O'Brien has the virus, becoming the highest-ranking U.S. official to test positive so far. The White House said he has mild symptoms, and "has been self-isolating and working from a secure location off site."
Also, Monday, the move to restart major league baseball ran into trouble just five days into the long-delayed season. Two ball games scheduled for Monday night were called off as the Miami Marlins coped with an outbreak.
Almost 4.3 million confirmed covid-19 cases have been reported in the United States, and there have been almost 148,000 deaths, according to Johns Hopkins University.
TRUMP, PENCE VISITS
President Donald Trump and Vice President Mike Pence each visited a vaccine development site Monday.
"I heard very positive things," Trump said when asked about the timetable for getting a vaccine to market, "but by the end of the year, we think we're in very good shape to be doing that."
Under the federal government's Operation Warp Speed program, multiple vaccines are being developed simultaneously with a goal of delivering 300 million safe and effective doses by January.
Trump visited the Fujifilm Diosynth Biotechnologies' Innovation Center in Morrisville, N.C., a suburb of Raleigh. That facility has begun production of the first batch of a possible vaccine developed by Novavax, a Maryland company.
The batches produced at the North Carolina facility will be used in a Phase 3 clinical trial of up to 30,000 participants, which is expected to begin this fall and will determine the drug's safety and effectiveness, according to Novavax, which has received $1.6 billion from the federal government.
Pence visited Miami to highlight the beginning of Phase 3 testing of the Moderna vaccine candidate.
"It's a historic day, a day when we begin in earnest to work on a vaccine," Pence said.
"We want to ensure we move at a safe and effective pace. I want to assure the people of Florida and people all across this country that we will cut no corners in the development of this or any vaccine," Pence said.
Company and government officials repeatedly underscored that while the vaccine effort is moving at record-breaking speed, safety is not being sacrificed.
"There is no compromise at all, with regard to safety, nor of scientific integrity," Fauci said.
"This is a significant milestone," National Institutes of Health Director Francis Collins said. "Yes, we're going fast, but no, we are not going to compromise" on proving whether the vaccine is safe and effective.
"We are focusing on speed because every day matters," added Stephane Bancel, CEO of Massachusetts-based Moderna.
MANY UNKNOWNS
Both vaccines starting testing Monday require two doses, spaced several weeks apart. Then researchers will have to wait to see if people get infected or sick from the coronavirus. What researchers hope to see is a clear benefit: fewer infections in people who received the vaccine, or less severe episodes of covid-19, the disease caused by the coronavirus.
There are many unknowns about how long it could take to get a clear sign of success or failure -- including how fast trial participants can be recruited and how long it takes for enough people to become infected to see whether there is an effect.
Statisticians have been crunching the numbers to predict how many infections would need to occur in the study population to gauge the vaccine's effectiveness. To show that the Moderna vaccine is 60% effective, Fauci said, there would need to be about 150 infections among the 30,000 participants.
The trials are also the biggest test yet of a technology that has never been approved for use outside medical research. Either vaccine could become the first in a new class of medicines.
The vaccines deliver a snip of genetic material that carries the blueprint for the spiky protein that dots the surface of the coronavirus. After a person is vaccinated, his cells would follow the genetic instructions to build the proteins, and his immune system, confronted with the spike protein, would learn to recognize it and defend against it without the person becoming infected.
"I believe it is a historic day: the first Phase 3 covid-19 vaccine being run in the U.S.," said Moderna's Bancel. "It's a historic day for science, as well. This is the first Phase 3 of a messenger RNA medicine in the world."
"We've been sitting on the sidelines passively attempting to wear our masks and social distance and not go out when it's not necessary. This is the first step of becoming active against this," said Dr. Frank Eder of Meridian Clinical Research, which runs a trial site in Binghamton, N.Y. "There's really no other way to get past this."
MEASURES ABROAD
The global tally by Johns Hopkins University shows more than 16.4 million confirmed cases of covid-19 and almost 652,000 deaths.
The World Health Organization said the pandemic continues to accelerate, with a doubling of cases over the past six weeks. The U.N. health agency's emergencies chief, Dr. Michael Ryan, stressed the need to "keep pressure on the virus."
"Every single country where pressure has been lifted on the virus, where virus is still at community level, there's been a jump back in cases," he said.
Europe's tourism revival is running into turbulence only weeks after countries reopened their borders. Rising infections in Spain and other nations are causing increasing concern among health authorities over people carrying the coronavirus home from their summer vacations.
European countries started opening to tourists in mid-June, but recent events have shown that the new freedom to travel is subject to setbacks.
Over the weekend, Britain imposed a 14-day quarantine on travelers arriving from Spain. Norway ordered a 10-day quarantine for people returning from the entire Iberian peninsula, and France urged its citizens not to visit Spain's Catalonia region.
In Austria, the lakeside resort town of St. Wolfgang shortened bar opening hours after an outbreak was detected Friday. By Monday, 53 people had tested positive, including many people working in the tourism industry.
In Germany, infections have been creeping higher, and officials decided last week to set up testing stations at airports to encourage people arriving from a long list of countries deemed high-risk -- including popular destinations such as Turkey -- to get tested. They will also allow people to get tested elsewhere for free within three days of arrival.
In Spain, the Catalonia and Aragon regions have the most worrying virus clusters, prompting authorities to tighten restrictions in Barcelona, in a rural area around Lleida and in Zaragoza that were relaxed only a month ago.
Catalonia is facing "the 10 most decisive days of this summer," regional leader Quim Torra said, warning that it is in everyone's hands to prevent a "critical situation" from worsening.
Belgium put the brakes on the country's coronavirus exit plan Monday with a set of drastic social distancing measures aimed at avoiding a new general lockdown as authorities in the province of Antwerp imposed a curfew amid a surge of infections.
Speaking after an urgent meeting of the national security council, Prime Minister Sophie Wilmes said contacts outside every household will be limited to the same five people over the next four weeks -- a "social bubble."
[Gallery not loading above? Click here for more photos » arkansasonline.com/728covid/]
"Our aim is clear -- avoid another full lockdown," he said.
Greek authorities said they are likely to extend the mandatory use of masks at churches and shopping malls.
[CORONAVIRUS: Click here for our complete coverage » arkansasonline.com/coronavirus]
And in North Africa, Morocco banned most travel to and from some major cities -- including Tangier, Casablanca and Marrakech -- to stem a small spike in cases.
In the Asia-Pacific region, many countries are still essentially banning foreign travelers or, if they do allow them to enter, requiring them to submit to tests and strict quarantine. That includes Australia, where the premier of Victoria state, Daniel Andrews, said the biggest driver in the region's outbreak is people continuing to go to work after showing symptoms.
The crossing of borders was linked to other outbreaks in Asia. South Korea said 16 of the 25 new cases it confirmed Monday were tied to people arriving from abroad. Over the past few days, the country reported dozens of cases among crew members of a Russia-flagged cargo ship and hundreds of South Korean construction workers airlifted from Iraq.
Information for this article was contributed by Carolyn Y. Johnson of The Washington Post; and by Lauran Neergaard, Michael Hill, Jocelyn Noveck, Zeke Miller, Kevin Freking, Jonathan Lemire, Geir Moulson, Elaine Kurtenbach, Samuel Petrequin and staff members of The Associated Press.