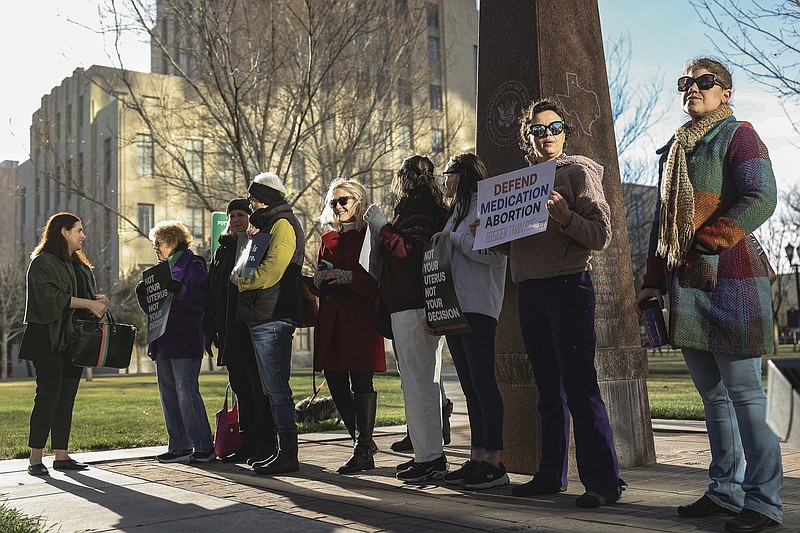AMARILLO, Texas -- A federal judge in Texas raised questions Wednesday about a Christian group's effort to overturn federal regulators' decades-old approval of a leading abortion drug, in a case that could threaten the country's most common method for ending pregnancies.
Judge Matthew Kacsmaryk heard more than four hours of debate over the Alliance Defending Freedom's request to revoke or suspend the Food and Drug Administration's approval of mifepristone. Such a step would be an unprecedented challenge to the FDA and its authority in deciding which drugs to permit on the market.
Kacsmaryk said he would rule "as soon as possible," without giving any clear indication of how he might decide and leaving open the question of whether access to the standard regimen for medication abortions might soon be curtailed throughout the country.
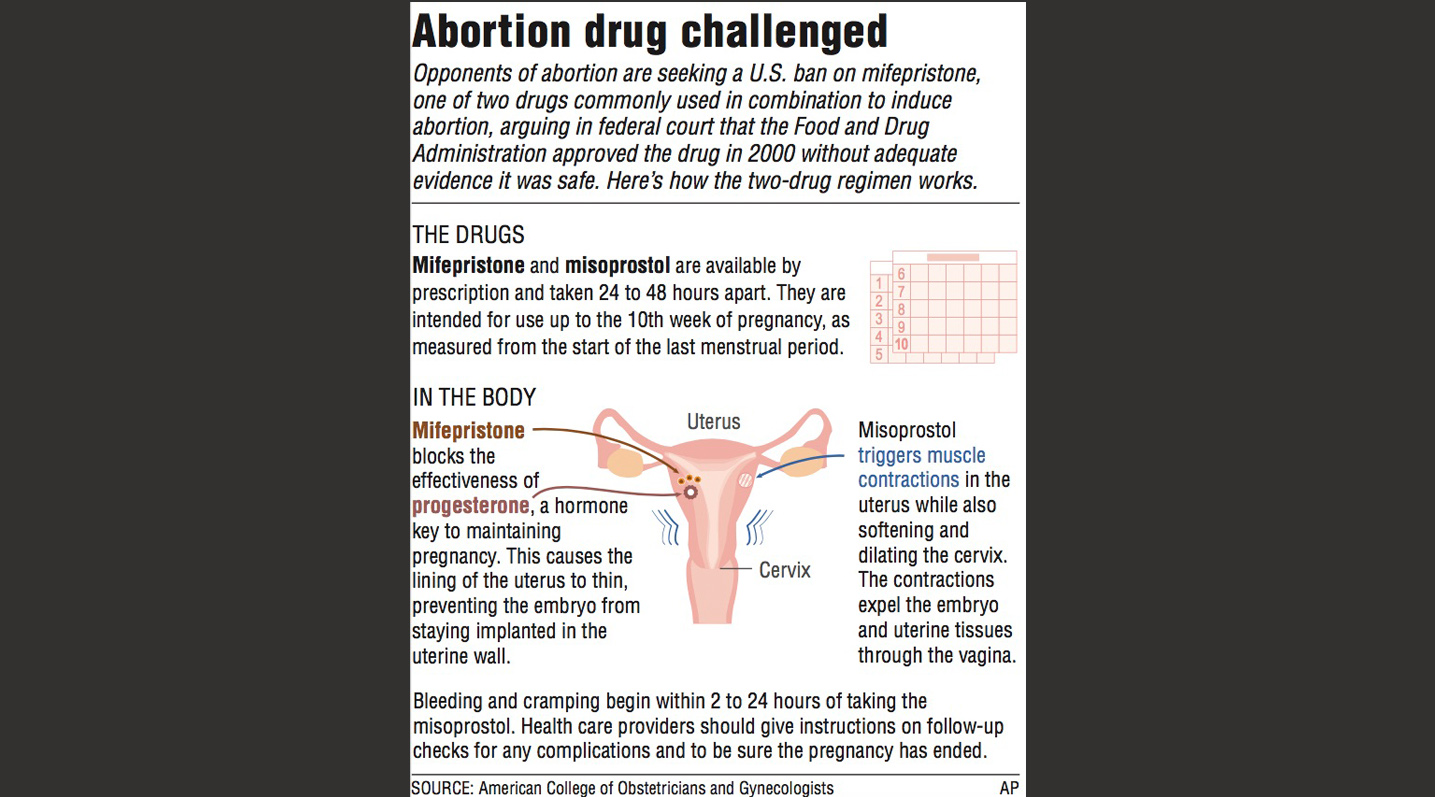
Mifepristone, when combined with a second pill, was approved in 2000 and is used to end pregnancies until their 10th week. It has been increasingly prescribed since last summer's U.S. Supreme Court ruling overturning Roe v. Wade.
The Texas lawsuit has become the latest high-stakes legal battle over access to abortion since the question of its legality was returned to the states.
Kacsmaryk, who was appointed by former President Donald Trump, saved some of his most pointed questions for attorneys representing the alliance.
"Explain to me why this court has that sweeping authority?" Kacsmaryk asked, in reference to the group's request to pull mifepristone from the market.
The judge also questioned whether the group had the legal standing to obtain a pretrial ruling, grilling both sides on U.S. Supreme Court cases that set out when such extraordinary relief is allowed.
Still, the judge also posed questions suggesting he was considering how he might draft a preliminary injunction in the plaintiffs' favor, at one point asking the alliance's lawyers if the issue of standing had been clearly addressed by appellate courts. At another point, he told them that their outline for the order of their arguments "tracks the elements for an injunction nicely."
Lawyers representing the FDA argued that pulling mifepristone would disrupt reproductive care for women across the U.S.
"An injunction here would interfere with the interests of every state in the country" said Julie Straus Harris of the U.S. Justice Department, which represented the FDA.
Straus Harris and her colleagues also questioned whether the alliance -- which filed its case on behalf of several anti-abortion doctors -- had standing to bring the lawsuit, given that none of the plaintiffs could show the type of harm typically needed for such a legal action.
Public health professionals and legal experts have denounced the lawsuit as unsupported by scientific evidence and said it could upend the FDA's overall drug approval process. The agency has repeatedly found the two-step medication abortion protocol to be a safe and effective alternative to surgical abortions.
Justice Department lawyers object to the court potentially second-guessing the FDA's technical expertise and have called the request for an injunction "extraordinary and unprecedented." The lawsuit, the government said in court filings, is based on false claims that the drug inflicts severe complications and "fails to acknowledge that the alternatives to mifepristone -- surgical abortion or continued pregnancy -- also have rates of complications, with childbirth's being substantially higher than mifepristone's."
THE ARGUMENTS
One of the chief arguments leveled against the FDA in the lawsuit is that the agency misused its authority when it originally approved the abortion pill.
The FDA reviewed the drug under its so-called accelerated approval program, which was created in the early 1990s to speed access to the first HIV drugs. Since then, it has been used to expedite drugs for cancer and other "serious or life-threatening diseases."
"The plain text is clear it applies to illnesses," argued Erik Baptist, the alliance's lead attorney. "Mifepristone is used to end pregnancies, and pregnancy isn't an illness."
The FDA rejected the group's argument on multiple accounts. First, attorneys said FDA regulations make clear that pregnancy is considered a "medical condition" that can be serious and life-threatening in some cases.
Second, government attorneys said the terms of mifepristone's use were replaced more than a decade ago by subsequent FDA programs passed by Congress, rendering the argument irrelevant.
Finally, while the FDA reviewed the drug under its accelerated approval regime, it didn't expedite the drug's review. In fact, approval only came after four years of deliberation. Instead, the FDA used regulatory powers under the accelerated program to add extra safety restrictions to mifepristone.
Legal experts have long been deeply skeptical of many of the arguments made by the alliance. And there is essentially no precedent for a lone judge overruling an FDA drug approval decision.
At one point, Kacsmaryk asked the alliance's attorneys about the possibility of suspending mifepristone's approval, without withdrawing it completely.
"Any relief you grant must be complete" and apply nationwide, Baptist said. "The harms of these abortion drugs know no bounds."
The judge pressed him about whether he could come up with an "analogue where courts have intervened in such a way" so many years after a drug has been approved.
"No, I can't," said Baptist. He noted that individuals spent years trying to challenge the drug's approval internally through the FDA.
Kacsmaryk also solicited guidance from Baptist on how to shape a possible order, asking whether the conservative group believes he could unilaterally direct the FDA to withdraw its approval, or just begin that process.
The court can "on its own accord" order the FDA withdrawal, Baptist said.
Kacsmaryk gave each side two hours to make their arguments -- with time for rebuttal -- in the high-stakes case. Mifepristone's manufacturer, Danco Laboratories, joined the FDA in arguing to keep the pill available.
A ruling could come any time. A decision against the drug would be swiftly appealed by the Justice Department, which would also likely seek an emergency stay to stop it from taking effect while the case proceeds.
Members of the Women's March advocacy group rallied outside the courthouse, including one dressed as a kangaroo to decry the proceedings as a "kangaroo court."
Ultimately, courthouse officials allowed 20 members of the media and 20 members of the general public to attend the hearing.
The case was filed by the Alliance for Hippocratic Medicine, an organization that lists five anti-abortion groups as its members, and four individual doctors who are against abortion rights. The group incorporated in Amarillo in August, shortly after the Supreme Court overturned Roe v. Wade. The case was assigned to Kacsmaryk, the only judge in the Northern District of Texas who covers the Amarillo division. Before his appointment by Trump, Kacsmaryk wrote an article that was critical of Roe v. Wade, as well as issues such as marriage equality and federal anti-discrimination protections for sexual orientation and gender identity.
If Kacsmaryk rules against the FDA, it's unclear how quickly access to mifepristone could be curtailed or how the process would work. The FDA has its own procedures for revoking drug approvals that involve public hearings and scientific deliberations, which can take months or years.
If mifepristone is sidelined, clinics and doctors that prescribe the combination say they would switch to using only misoprostol, the other drug used in the two-drug combination. That single-drug approach has a slightly lower rate of effectiveness in ending pregnancies but is widely used in countries where mifepristone is illegal or unavailable.
However Kacsmaryk rules, his decision will likely be appealed to the conservative U.S. Court of Appeals for the 5th Circuit and the case could eventually reach the Supreme Court.
Much of Wednesday's hearing focused on federal regulations and FDA processes. It did not delve into the legality of abortion or when life begins.
In a two-step medication abortion, a patient first takes one mifepristone pill, which terminates the pregnancy. Approximately 24 hours later, they typically take a four-pill dose of misoprostol, a drug introduced in 1973 to treat stomach ulcers, to soften the cervix and prompt contractions that expel the embryo or fetus. While misoprostol is widely used on its own to perform abortions around the world, studies show it is less effective than the two-step regimen, and usually causes more cramping and bleeding.
Without access to mifepristone, many providers say, they would continue to offer medication abortions using only misoprostol. Some would perform only surgical abortions. Providers said either scenario would result in major upheaval as they try to enact new procedures, which some clinic owners fear may present legal hurdles. A few have been stockpiling mifepristone in anticipation of the ruling, hoping they would still be permitted to distribute the pills they already have regardless of Kacsmaryk's decision.
In addition to challenging mifepristone's approval process, the lawsuit takes aim at several later FDA decisions that loosened restrictions on the pill, including eliminating a requirement that women pick it up in person.
The FDA has regulated mifepristone more stringently than many other drugs. For a dozen years, the agency has imposed an additional framework of restrictions and monitoring for the drug. Called a Risk Evaluation and Mitigation Strategy, or REMS, it has been used for only about 300 other drugs, only 60 of which are actively under the framework.
In recent years, the FDA has extensively reviewed new data on mifepristone and has lifted several of the restrictions, including the requirement that patients obtain the drug in person from a provider.
Some of the same anti-abortion organizations that filed the Texas lawsuit had previously filed, in 2002 and 2019, citizen petitions opposing the FDA's actions on mifepristone. Both were rejected by the agency as unfounded. And a 2008 review by the Government Accountability Office found no irregularities with the FDA's mifepristone approval.
Information for this article was contirbuted by Sean Murphy, Matthew Perrone and Jake Bleiberg of The Associated Press; by Perry Stein, Caroline Kitchener and Ann E. Marimow of The Washington Post; and by Pam Belluck and Allison McCann of The New York Times.
 A security guard keeps an eye outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson)
A security guard keeps an eye outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson) Front of federal court building is shown on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson)
Front of federal court building is shown on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson) Three members of the Women's March group protest in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson)
Three members of the Women's March group protest in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson) FILE - Bottles of the drug misoprostol sit on a table at the West Alabama Women's Center, March 15, 2022, in Tuscaloosa, Ala. Misoprostol induces uterus contractions that expel an embryo or fetus and other tissue. A federal judge in Texas will hear arguments Wednesday, March 15, 2023, in a high-stakes court case that could threaten access to abortion medication and blunt the authority of U.S. drug regulators. The lawsuit from Christian conservatives aimed at overturning the Food and Drug Administration's approval of the abortion pill mifepristone. The drug, when used with a second pill, misoprostol, has become the most common method of abortion in the U.S. (AP Photo/Allen G. Breed, File)
FILE - Bottles of the drug misoprostol sit on a table at the West Alabama Women's Center, March 15, 2022, in Tuscaloosa, Ala. Misoprostol induces uterus contractions that expel an embryo or fetus and other tissue. A federal judge in Texas will hear arguments Wednesday, March 15, 2023, in a high-stakes court case that could threaten access to abortion medication and blunt the authority of U.S. drug regulators. The lawsuit from Christian conservatives aimed at overturning the Food and Drug Administration's approval of the abortion pill mifepristone. The drug, when used with a second pill, misoprostol, has become the most common method of abortion in the U.S. (AP Photo/Allen G. Breed, File) Homeland Security officers secure the scene outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson)
Homeland Security officers secure the scene outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson) Lindsay London holds protest sign in front of federal court building in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson)
Lindsay London holds protest sign in front of federal court building in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson) In this image from video from the Senate Judiciary Committee, Matthew Kacsmaryk listens during his confirmation hearing before the Senate Judiciary Committee on Capitol Hill in Washington, on Dec. 13, 2017. U.S. District Judge Matthew Kacsmaryk is holding a hearing in a case that could throw into jeopardy access to the nation's most common method of abortion. He is a former attorney for a Christian legal group who critics say is being sought out by conservative litigants because they believe he'll be sympathetic to their causes. (Senate Judiciary Committee via AP)
In this image from video from the Senate Judiciary Committee, Matthew Kacsmaryk listens during his confirmation hearing before the Senate Judiciary Committee on Capitol Hill in Washington, on Dec. 13, 2017. U.S. District Judge Matthew Kacsmaryk is holding a hearing in a case that could throw into jeopardy access to the nation's most common method of abortion. He is a former attorney for a Christian legal group who critics say is being sought out by conservative litigants because they believe he'll be sympathetic to their causes. (Senate Judiciary Committee via AP) A Homeland Security Officer secures the scene outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson)
A Homeland Security Officer secures the scene outside the federal courthouse on Wednesday, March 15, 2023, in Amarillo, Texas, where a federal judge is expected to hear arguments in a lawsuit that takes aim at medication abortions. Pills are the most common method for obtaining an abortion in the U.S. (AP Photo/David Erickson) Lindsey London stands in protest in front of Federal Court Building in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson)
Lindsey London stands in protest in front of Federal Court Building in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. A conservative federal judge heard arguments Wednesday from a Christian group seeking to overturn the Food and Drug Administration's more than 2-decade-old approval of an abortion medication, in a case that could threaten the most common form of abortion in the U.S. (AP Photo/David Erickson)Gallery: Future of abortion drug in balance