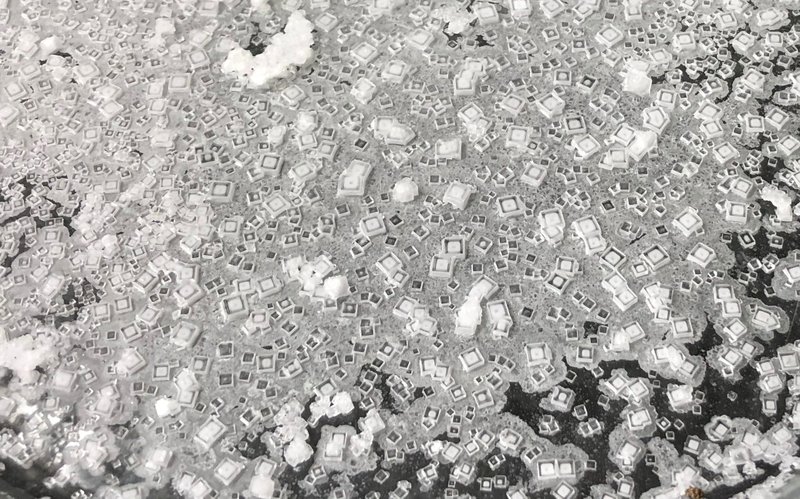
Humans have been harvesting salt since prehistoric times. In this kitchen-science experiment from ATK Kids — the children's website of America's Test Kitchen — chunky flakes of salt appear as a shallow "salty sea" slowly evaporates.
It's an easy project for elementary school kids, but that doesn't mean it's foolproof. Here are a few pro tips from an adult who tried becoming a salt farmer:
◼️ Don't just eyeball the measurements. If you use too little water, some of the salt you've measured won't go into solution. Use too much and you'll have to wait longer to see salt forming.
◼️ Preparing the saltwater for this project requires a lot of stirring. If you decide to speed up the process by dumping all the salt into the water before you heat it in the microwave, you will end up stirring longer than if you followed the directions.
◼️ If the water cools before you have stirred all the salt into solution because you took a phone call in the middle of your stirring, you can reheat the water in the microwave.
◼️ If you take a photo of the salt crystals as they grow, you can magnify the image to show off your salt to friends on social media.
◼️ Don't set the dish of salty water on your cat's favorite windowsill to evaporate.
THE BASICS
Safety: uses the microwave
Difficulty: beginner
Time: 10 minutes, plus how many days evaporation takes, which depends on how carefully you measure and how humid your house is.
Yield: about 1 tablespoon of salt
Gather these ingredients:
¼ cup distilled or filtered water
2 tablespoons kosher salt OR 1 tablespoon table salt
The recipe calls for distilled or filtered water because tap water can contain dissolved minerals that will be left behind when the water evaporates. These minerals are safe to eat, but they can give your salt a bitter flavor. Also, tap water might not be able to hold as much salt as distilled water will.
If you don't have filtered or distilled water, use tap water -- to see what happens. If the salt tastes bitter, you will know why.
The experts at America's Test Kitchen say less table salt is needed than kosher salt because the grains of table salt are so much smaller than the grains of kosher salt. Kosher salt is preferable to table salt because table salt often has anti-caking agents added to it, which will make the saltwater solution look cloudier and might also give the finished salt a bitter taste -- although it's perfectly safe to eat.
Gather this equipment:
Liquid measuring cup (microwave safe)
Oven mitts
1-teaspoon measuring spoon
Spoon
Coffee filter
8-inch square glass baking dish or pie plate
Hand lens (optional)
Get started:
Measure ¼ cup distilled or filtered water into measuring cup. Heat cup in microwave until water steams, 1 to 1½ minutes. Use oven mitts to remove the cup from the microwave (ask an adult for help).
Add 1 teaspoon of kosher salt to the hot water. Stir with spoon until salt is dissolved and water is clear. Continue adding salt, 1 teaspoon at a time and stirring, until all salt is dissolved into water.
If there are undissolved salt crystals after you've added all the salt and you are worn out from stirring, strain the solution through the coffee filter before continuing.
Cloudy water = undissolved salt
Clear water = salt has dissolved
Set the baking dish in a place where it won't be disturbed. (A warm, sunny spot will speed evaporation.) Carefully pour saltwater into baking dish -- this is your model of the salty sea.
Leave the dish undisturbed until all the water has evaporated. Use the hand lens to observe your model sea a few times as it evaporates.
Harvest your salt.
Use spoon to gently scrape salt crystals from bottom of baking dish. Salt can be stored in an airtight container indefinitely.
This flaky, chunky salt is great for sprinkling on finished dishes, such as eggs, vegetables, fruits, meats and more.
FOOD FOR THOUGHT
The hotter the water, the more salt you can dissolve into it.
The square or rectangular salt crystals harvested from a windowsill salt farm came from the much smaller crystals of kosher salt you started with. When you dissolve salt in water the salt crystals separate into tiny ions. When dissolved salt ions find one another, they lock together and fall out of the solution. As more salt joins them, each crystal grows bigger and bigger.
The more slowly a salt solution cools, the more time the salt ions have to find one another, growing larger crystals.
SALT'S ORIGIN STORY
All salt comes from the sea. Some comes from the oceans that cover most of the earth today. Other salt comes from underground deposits left behind when ancient oceans dried up millions of years ago.
According to America's Test Kitchen, here are the two main ways that manufacturers harvest salt:
◼️ They pump water into underground deposits to dissolve the salt. Then machines pump the salty water mixture out of the ground and into big tanks, which heat up. The heat causes the water to evaporate, leaving the salt behind.
◼️ To harvest salt from the ocean, manufacturers create vast but shallow ponds of seawater. Over time, the water evaporates and big salt crystals sink to the bottom. Manufacturers rake up the salt. Some sea salt is made from seawater that's heated to speed up evaporation.
ADG Families on 05/30/2020